I am considering building a cell-checker pressure pot as well. Many thanks for sharing your experiences in this thread.
How do you account from the fact that the vessel initially has air and the fraction of O2 changes as you increase the pressure?
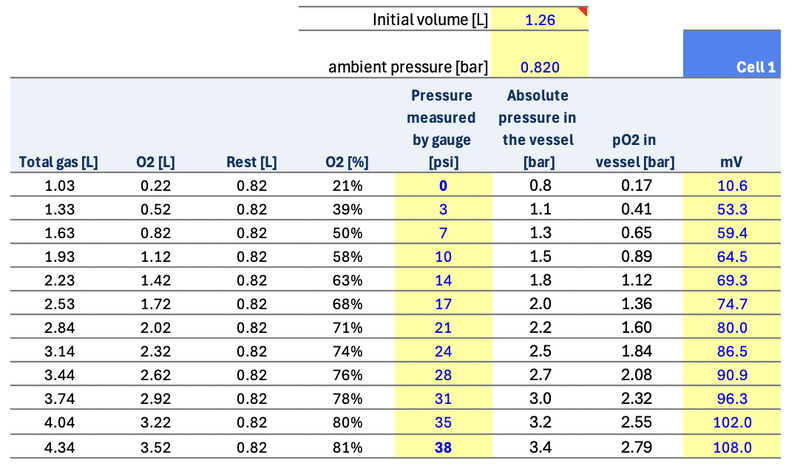
I did some quick calculations below to simulate going from 0 to 38 psi measured by the gauge by adding O2 to a vessel of 1.26 L of volume initially filled with air at an ambient pressure of 820 mbar. The fraction of O2 goes from 21% (air at the beginning) to 81% when reaching 38 psi by adding O2. The pO2 in the vessel is calculated based on absolute pressure and fraction of O2 in the vessel.
How do you account from the fact that the vessel initially has air and the fraction of O2 changes as you increase the pressure?
I did some quick calculations below to simulate going from 0 to 38 psi measured by the gauge by adding O2 to a vessel of 1.26 L of volume initially filled with air at an ambient pressure of 820 mbar. The fraction of O2 goes from 21% (air at the beginning) to 81% when reaching 38 psi by adding O2. The pO2 in the vessel is calculated based on absolute pressure and fraction of O2 in the vessel.



