Sorry if this is the wrong place for this. I am going for my Nitrox certification, and someone had mentioned the Circle-T formula. I have read the book and I do not see where that formula is mentioned. I know about the EAD, O2 PP, Max Depth and Contingency Depth. Is there another name that is goes by. Thanks.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Nitrox Question
- Thread starter scuba_frog
- Start date
Please register or login
Welcome to ScubaBoard, the world's largest scuba diving community. Registration is not required to read the forums, but we encourage you to join. Joining has its benefits and enables you to participate in the discussions.
Benefits of registering include
- Ability to post and comment on topics and discussions.
- A Free photo gallery to share your dive photos with the world.
- You can make this box go away
I'm not sure if it's the same thing, but they are probably referring to Dalton's diamond, which is just a short hand way to figure out the formulas.
Ok, that's a new one too. I'm supposed to take the written test tonight. I know the theory (I think). Those formulas simple when they are laid out in front of you.
nereas
Contributor
- Messages
- 2,735
- Reaction score
- 9
- # of dives
- 500 - 999
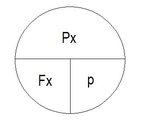
Yup, sounds like Dalton's diamond (from Dalton's gas law):
pp
____________________
fraction | total-pressure
You put your thumb over the value you are seeking, and the diagram tells you how to derive it from the other two.
Example 1:
Cover pp with your thumb, and the diagram tells you that pp = fraction x total pressure
Example 2:
Cover fraction with your thumb, and the diagram tells you that fraction = pp divided by total pressure.
Example 3:
Cover total-pressure with your thumb, and the diagram tells you that total pressure = pp divided by fraction.
Total pressure equals (depth in seawater divided by 33 ft/ATA) + 1 ATA
Fraction equals the percentage fraction of the component gas (either oxygen, or nitrogen, or helium) in the gas mix (either air, or nitrox, or trimix).
PP equals the partial pressure of the component gas (either oxygen, or nitrogen, or helium) in the gas mix (either air, or nitrox, or trimix).
Note that for simplicy we always combine the argon and other fractional components with the nitrogen.
The T diagram helps you to remember how the formulas/formulae work. You are right, that they are simple when they are laid out in front of you.
These formulae are primarily useful to determine your "best mix" for deep diving with trimix. They are normally just introduced in the basic nitrox course, then elaborated upon more in advanced nitrox, which is often taught in conjunction with a basic decompression diving course.
For your basic nitrox diving, if you just remember that EAN 32 is great for diving to 130 fsw, and that EAN 36 is great for diving to 95 ft, and then dive accordingly, then you will do fine. Of course, the test will probably be a lot harder than that.
pp
____________________
fraction | total-pressure
You put your thumb over the value you are seeking, and the diagram tells you how to derive it from the other two.
Example 1:
Cover pp with your thumb, and the diagram tells you that pp = fraction x total pressure
Example 2:
Cover fraction with your thumb, and the diagram tells you that fraction = pp divided by total pressure.
Example 3:
Cover total-pressure with your thumb, and the diagram tells you that total pressure = pp divided by fraction.
Total pressure equals (depth in seawater divided by 33 ft/ATA) + 1 ATA
Fraction equals the percentage fraction of the component gas (either oxygen, or nitrogen, or helium) in the gas mix (either air, or nitrox, or trimix).
PP equals the partial pressure of the component gas (either oxygen, or nitrogen, or helium) in the gas mix (either air, or nitrox, or trimix).
Note that for simplicy we always combine the argon and other fractional components with the nitrogen.
The T diagram helps you to remember how the formulas/formulae work. You are right, that they are simple when they are laid out in front of you.
These formulae are primarily useful to determine your "best mix" for deep diving with trimix. They are normally just introduced in the basic nitrox course, then elaborated upon more in advanced nitrox, which is often taught in conjunction with a basic decompression diving course.
For your basic nitrox diving, if you just remember that EAN 32 is great for diving to 130 fsw, and that EAN 36 is great for diving to 95 ft, and then dive accordingly, then you will do fine. Of course, the test will probably be a lot harder than that.
nereas
Contributor
- Messages
- 2,735
- Reaction score
- 9
- # of dives
- 500 - 999
Never heard of a circle-T formula either and i teach nitrox for 2 agencies and trained under a third.
Obviously not for any "technical" agencies.

Blackwood
Contributor
nereas
Contributor
- Messages
- 2,735
- Reaction score
- 9
- # of dives
- 500 - 999
It's a way of writing Px=Fx*p.
It's used as a teaching aid in other algebraic situations as well (such as Ohm's law), but I fail to see how it's helpful in any way.
You need to use your thumb, like I said above.
Obviously not for any "technical" agencies.
TDI dont count?
Edit:- it appears to be a strange way of working out the formula. Ive seen it refereed to as Ohms Law triangle before but never anyones circle.
It's a way of writing Px=Fx*p.
It's used as a teaching aid of other algebraic relationships as well (such as Ohm's law), but I fail to see how it's helpful in any way.
Edit:- it appears to be a strange way of working out the formula. Ive seen it refereed to as Ohms Law triangle before but never anyones circle.
Agreed- It's a good way to start, but once you understand the theory, it's faster and less complicated to just go straight to the calculations.
Similar threads
- Replies
- 34
- Views
- 2,052