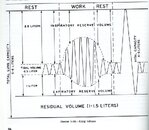
I've read a lot here, but I'm not sure how much to believe. This U.S. Navy Diving Manual drawing (Figure 1-24, Lung Volume, from the March 1970 edition on page 1.3.4) shows how many people breathe. If a diver simply uses his or her tidal volume, the respirations are much more frequent and pass less air (average about half a liter). But we have a lot more vital capacity than that, up to 4-5 liters (mine is 6+ liters, as measured by spirometry on a respirator physical for work). In diving, we want to increase the breathing depth to include both some of our inspiratory reserve volume, anhd our expiratory reserve volume, as is shown in the center of the diagram for the "work" phase of breathing. This increases the efficiency of breathing, decreases the possibility of CO2 buildup, and actually decreases air consumption as the air breathed is more efficiently used. You can read more in the current U.S. Navy Diving Manual at this link:I'm trying to improve my air consumption. I'm always the air hog and the dive ends because of me. I usually do breathe from my diaphragm and I try to slow things down as much as I can, but I was reading recently some tips and ran across this aritcle on scubadiving.com which says:
The most important thing I was taught was that I should *never* hold my breath, but this is basically telling me that I should "pause" for a few seconds (three?) before exhaling, which seems to me to basically be holding my breath. This would certainly slow down my breathing, because I was trying to keep a constant circular flow going, and just try and slow down the exhale and inhale parts by doing it as slowly as I could.
- Pause after inhaling. Use your diaphragm to hold air in your lungs a few extra seconds while keeping your throat open. This allows even more time for gas exchange. Your breathing pattern should be: Exhale, inhale, pause. Exhale, inhale, pause.
Note: Every time we describe this breathing pattern, someone writes us, "Isn't this skip breathing?" It's not. Skip breathing involves holding your breath by closing your epiglottis (like when you grunt) and holding it for much longer. Closing your throat creates a closed air space that is vulnerable to embolism if you ascend. Keeping your throat open avoids that risk. Besides, skip breathing doesn't work. Holding your breath too long means retaining too much carbon dioxide, triggering the urge to breathe sooner than necessary and resulting in rapid shallow breathing. The net result: You use more air by skip breathing, not less.
Do other people do this? Is this why I'm always dragging people back to the surface when they still have twice the air left as I do? Do you have other tips?
http://www.navsea.navy.mil/Portals/103/Documents/SUPSALV/Diving/US DIVING MANUAL_REV7.pdf?ver=2017-01-11-102354-393
Here is what this latest version of the manual states about air consumption:
I would not fixate on improving air consumption, but rather try to relax and enjoy diving. I used to dive our jump tanks (twin 42s) with other PJs diving twin 72s, and I would stay about twenty feet above them and have about the same time in the water.3-4.8 Oxygen Consumption. A diver’s oxygen consumption is an important factor when determining how long breathing gas will last, the ventilation rates required to maintain proper helmet oxygen level, and the length of time a canister will absorb carbon dioxide. Oxygen consumption is a measure of energy expenditure and is closely linked to the respiratory processes of ventilation and carbon dioxide production.
Oxygen consumption is measured in liters per minute (l/min) at Standard Temperature (0°C, 32°F) and Pressure (14.7 psia, 1 ata), Dry Gas (STPD). These rates ofoxygen consumption are not depth dependent. This means that a fully charged MK 16 oxygen bottle containing 360 standard liters (3.96 scf) of usable gas will last 225 minutes at an oxygen consumption rate of 1.6 liters per minute at any depth, provided no gas leaks from the rig.
Minute ventilation, or respiratory minute volume (RMV), is measured at BTPS (body temperature 37°C/98.6°F, ambient barometric pressure, saturated with water vapor at body temperature) and varies depending on a person’s activity level, as shown in Figure 36. Surface RMV can be approximated by multiplying the oxygen consumption rate by 25. Although this 25:1 ratio decreases with increasing gas density and high inhaled oxygen concentrations, it is a good ruleofthumb approximation for computing how long the breathing gas will last.
Unlike oxygen consumption, the amount of gas a diver inhales is depth dependent. At the surface, a diver swimming at 0.5 knot inhales 20 l/min of gas. A SCUBA cylinder containing 71.2 standard cubic feet (scf) of air (approximately 2,000 standard liters) lasts approximately 100 minutes. At 33 fsw, the diver still inhales 20 l/min at BTPS, but the gas is twice as dense; thus, the inhalation would be approximately 40 standard l/min and the cylinder would last only half as long, or 50 minutes. At three atmospheres, the same cylinder would last only onethird as long as at the surface.
Carbon dioxide production depends only on the level of exertion and can be assumed to be independent of depth. Carbon dioxide production and RQ are usedto compute ventilation rates for chambers and free-flow diving helmets. Thesefactors may also be used to determine whether the oxygen supply or the duration of the CO2 absorbent will limit a diver’s time in a closed or semiclosed system.
'Hope this helps.
SeaRat