If you can measure the temperature you can also skip the "cool down" step and enter the measured temperature into BB instead after each step. That'll give you turn by turn instructions that ideally end in the same place as your longer process.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Problem with Baltic Blender, wrong calculation
- Thread starter Agro
- Start date
Please register or login
Welcome to ScubaBoard, the world's largest scuba diving community. Registration is not required to read the forums, but we encourage you to join. Joining has its benefits and enables you to participate in the discussions.
Benefits of registering include
- Ability to post and comment on topics and discussions.
- A Free photo gallery to share your dive photos with the world.
- You can make this box go away
I believe it uses the specified initial temp for source and destination cylinders. Source pressure doesn't really figure into it.Does it know the pressure/temperature of the gas being added?
The calculations are not assuming "best-guess" anything. The calculations are based on what you are setting as the measured gas temperature and measured pressure. If you want to be that exact, then you do need to allow the cylinder and gas to reach thermal equilibrium at each step of the process. I find that using an infrared temperature reader on the sidewall of the cylinder tends to be enough accuracy for setting the temperature. I fill outside, so my ambient temperature can change during the daySevenrider, that's it, thank you very much. I must change temperature in settings to 14.5 as well. Otherwise it assumes some best-guess temperature changings.
I blend this way:
- add oxygen to 202 bar, cylinder gets a little bit hotter
- let it cool down to 14.5 celsius
- measure, for ex 200bar
- add oxygen to 201, hardly any changing in temp.
- add air to 252 bar
- let it cool down
- measure, for ex 248 bar
- add air to 250 bar
This is the only way to have a constant temperature and therefore no mistake by temperature. Off course you need time to practice this kind of blending.
If having 21 degrees and 14.5 degrees the programm seems to assume you make a fast filling and starts best-guess calculation. No idea what parameters it takes:
- Filling speed?
- Temperatur of oxygen and air? Coming out of cylinder is much colder then out of a compressor or booster.
- Steel or alu tank? Different heat conduction.
Anyway if I have to make a fast filling perhaps program's best-guess-calculation is better then mine
I (and most people I know) leave the "Standard Temperature" at 70 Fahrenheit (21 Celsius) all the time. This is the temperature for the pressure rating stamped on the cylinder (I believe that is true in Europe...but I don't know). Guy-Lussac's law tells me that if the temperature of the gas in the cylinder is higher or lower than 21 Celsius that the pressure of a "full" cylinder will change. A 250 bar rated cylinder would be considered "full" at 226 bar if the gas temperature is 2 Celsius. That same cylinder would be considered "full" at 269 bar if the gas temperature is 35 Celsius.
The application will adjust the fill pressures higher or lower based on temperature to match the desired pressure at standard temperature. The temperature section in the application Help page goes into a lot of detail.
No idea how to measure the temperature of the gas(!), I can only measure the cylinder's outside temperature, this migth be far away from gas temperature inside the cylinder.If you can measure the temperature you can also skip the "cool down" step and enter the measured temperature into BB instead after each step. That'll give you turn by turn instructions that ideally end in the same place as your longer process.
Actually it is not that hard unless you're in a hurry. Once the gas and the cylinder reach thermal equilibrium, you measure the outside of the cylinder.No idea how to measure the temperature of the gas(!), I can only measure the cylinder's outside temperature, this migth be far away from gas temperature inside the cylinder.
Seems like you have to repeatedly measure the outside cylinder temp to know when thermal equilibrium has been reached.Once the gas and the cylinder reach thermal equilibrium, you measure the outside of the cylinder.
If you want to be exact and match the output of the program, yes. I wait a few minutes, measure the temperature of the tank, adjust the temperature in the mixer app to what I measured if needed, top off to the pressure, and move on. The OP is using Baltic Blender and the App has a help menu. This is all explained in the app itself.Seems like you have to repeatedly measure the outside cylinder temp to know when thermal equilibrium has been reached.
Sounds good but I am sure, gas and cylinder wall never ever have equilibium, unless gas and wall and outside gas have the same temp.If you want to be exact and match the output of the program, yes. I wait a few minutes, measure the temperature of the tank, adjust the temperature in the mixer app to what I measured if needed, top off to the pressure, and move on. The OP is using Baltic Blender and the App has a help menu. This is all explained in the app itself.
If you have 40 celsius inside the cylinder and 10 celsius outside the cylinder, then you have less then 40 celsius at wall's inside and at wall's outside you have anything warmer then 10. Heat is coming out of the cylinder so wall must have a temp difference between inside and outside, otherwise wall would be a perfect temp isolator, which it is not.
I can imagine that wall's outside temp is closer to inside gas' temp then to outside gas temp. But it is never ever the same, unless full equilibrium (in, wall, out).
So question is how much is difference between inside gas temp and wall's outside temp. Obviously it is not that much otherwise calculation can not work. And according to Sevenrider it seems to work.
- Messages
- 14,180
- Reaction score
- 11,412
- Location
- Port Orchard, Washington State
- # of dives
- 1000 - 2499
well kinda?Rjack, I thougt you were making a joke. It seems I was wrong, sorry for that.
Since you had the right mix but the pressure was low and temperature seems "cool" for a final cylinder temp. So I just used P1/T1 = P2/T2 to figure out how much warmer it would need to be to reach 250bar. Not sure where in the app it's using temperature or why you're getting 21C when Guy-Lussac's gives me 24C (yes I used Kelvin)
I still have my problems with baltic blender's temperature. I put everything to 14 celsius.
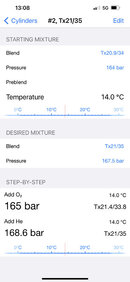
Starting mix is 20.0/34.0, 164 bar.
Desired mix is 21/35, 167.5 bar
Tool tells me to add O2 up to 165 bar and He up to 168.6 bar.
As far as I understood the discussion so far I must add gases (O2 and He) up to 168.6 bar HOT (what ever hot is) to get 167.5 bar at 14 celsius. Or am I wrong?
But what about the O2? Do I have to add up to 165.0 hot? Or do I have to wait until I have 14 celsius at 165 bar cold?
Still I am confused......
Starting mix is 20.0/34.0, 164 bar.
Desired mix is 21/35, 167.5 bar
Tool tells me to add O2 up to 165 bar and He up to 168.6 bar.
As far as I understood the discussion so far I must add gases (O2 and He) up to 168.6 bar HOT (what ever hot is) to get 167.5 bar at 14 celsius. Or am I wrong?
But what about the O2? Do I have to add up to 165.0 hot? Or do I have to wait until I have 14 celsius at 165 bar cold?
Still I am confused......
Attachments
Similar threads
- Replies
- 16
- Views
- 1,595
- Replies
- 4
- Views
- 669
- Replies
- 9
- Views
- 1,802
- Replies
- 67
- Views
- 10,678