Ok, if you want to look at it that way you still get the same result. The ppO2 in the blood is lower at 3m than 6m while the ppN2 in the tissues start out the same, thus there is a greater pressure differential at 3m and faster movement of the N2 to the blood.Exactly, which is included in the partial pressure.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Increased nitrogen off-gassing 10ft/3m VS 20ft/6m on 100% oxygen
- Thread starter ScubaSamScubaStevesFather
- Start date
Please register or login
Welcome to ScubaBoard, the world's largest scuba diving community. Registration is not required to read the forums, but we encourage you to join. Joining has its benefits and enables you to participate in the discussions.
Benefits of registering include
- Ability to post and comment on topics and discussions.
- A Free photo gallery to share your dive photos with the world.
- You can make this box go away
Blood is a tissue with a non-zero half life. The tissue tension doesn't drop instantly when you ascend. It decays exponentially, eventually reaching the partial pressure of the inspired inert gas. When breathing O2, that inspired partial pressure is zero at all depths. The time constant of that decay (i.e., the tissue half-life) is independent of pressure.
For tissue loading to be less at 3 m than at 6m, I think at least one of those statements has to be wrong. However, I'm hard-pressed to see which or why that would be the case.
For tissue loading to be less at 3 m than at 6m, I think at least one of those statements has to be wrong. However, I'm hard-pressed to see which or why that would be the case.
admikar
Contributor
Makes sense, except you are forgeting that total pressure is dropping in tissues too, hence bubble formation. By your logic, if you breathe air at 10 ft and go down to 20ft for pure O2, you wouldn't offgas nitrogen at all.The DCS issue aside, it's the basic physics of diffusion that the greater the pressure difference between two volumes, the faster that molecules will move from the area of high pressure to the area of low pressure.
Other factors that affect diffusion speed are temperature, the relative concentration of different molecules, surface area and membrane porosity. But in this case we are holding all of those constant. It's only the pressure differential that varies.
Specifically, the two volumes are the dissolved gases in the tissue and the surrounding blood. The gases in the blood are very near ambient while the gases in the tissue are at some higher pressure. As we ascend, the pressure differential between the gases in the tissue and the blood increases and thus the speed in which the N2 moves from the former to the latter increases.
The very fastest offgassing plan is always to ascend straight to the surface and then breathe 100% O2. Of course that ignores the whole reason you are trying to offgass N2 which is to reduce the likelihood of DCS. Still if you concerned about minimizing your N2 load for the next dive(s), doing your final O2 stop at 3m instead of 6m would have a small, but real, advantage.
If this doesn't show up in the results from a particular decompression algorithm, then that shows the programmer or person creating the algorithm has made simplifying assumptions, not that the physics is invalid.
I'm not forgetting that. I have mentioned it repeatedly. But for the purpose of this theoretical discussion, we are deliberately ignoring it.Makes sense, except you are forgeting that total pressure is dropping in tissues too, hence bubble formation.
That's incorrect. There is still a greater concentration of N2 molecules in the tissues than the blood, so they would continue to diffuse from the former to the latter. Just at a lower rate than if you breathed your air at 10ft and then went to 100% O2 at the surface.By your logic, if you breathe air at 10 ft and go down to 20ft for pure O2, you wouldn't offgas nitrogen at all.
@lowwall, I usually hate to appeal to authority, but your contention is contrary to the works of Haldane, Workman, Schreiner, Baker, et. al. This is Baker's introductory comments, highlighting mine to emphasize he says the inert gas pressures, not the total gas pressures:
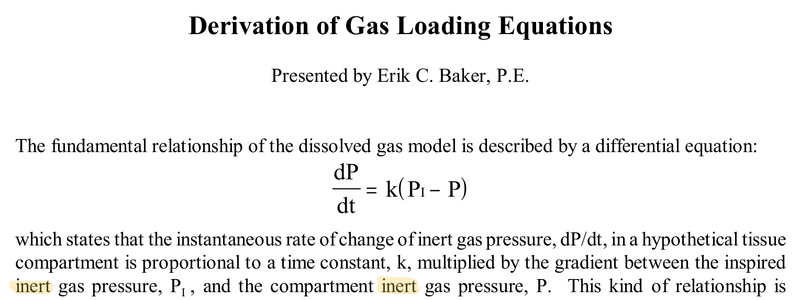
The solution of this with an ascent rate (possibly zero) is the Schreiner equation, which reduces to a simple exponential when breathing oxygen regardless of that rate. I suppose one could argue all those researchers made a simplifying assumption contrary to the real-world physics by starting with this differential equation, but I think we'll have to agree to disagree on this one.
For the record, if there is any nitrogen in the mix you're breathing, I absolutely agree with you that shallower is faster.
The solution of this with an ascent rate (possibly zero) is the Schreiner equation, which reduces to a simple exponential when breathing oxygen regardless of that rate. I suppose one could argue all those researchers made a simplifying assumption contrary to the real-world physics by starting with this differential equation, but I think we'll have to agree to disagree on this one.
For the record, if there is any nitrogen in the mix you're breathing, I absolutely agree with you that shallower is faster.
Blood is a tissue with a very low half-life. Going back to the factors that drive diffusion, surface area is the key. Hold on while I google "surface area alveoli"... OK. First result:Blood is a tissue with a non-zero half life. The tissue tension doesn't drop instantly when you ascend. It decays exponentially, eventually reaching the partial pressure of the inspired inert gas. When breathing O2, that inspired partial pressure is zero at all depths. The time constant of that decay (i.e., the tissue half-life) is independent of pressure.
For tissue loading to be less at 3 m than at 6m, I think at least one of those statements has to be wrong. However, I'm hard-pressed to see which or why that would be the case.
As it moves through blood vessels (capillaries) in the alveoli walls, your blood takes the oxygen from the alveoli and gives off carbon dioxide to the alveoli.
These tiny alveoli structures taken all together form a very large surface area to do the work of your breathing, both when you’re at rest and when you are exercising. The alveoli cover a surface that measures more than 1,076.4 square feet (100 square meters).
I find it helpful to simplify the model when I'm thinking about things like this. It often also helps to make the example more extreme. Here's my thought experiment. I have a tissue loaded with 4atm (absolute) of dissolved gases of which 3.2atm are N2. I've got a pair of capillaries with blood flowing through the tissue. The dissolved gases in both capillaries are 100% 02, but one capillary is at 4atm while the other is at 1atm.
Will there be a difference in how quickly N2 diffuses from the tissue into the two capillaries? To me the answer is an obvious yes, driven by the difference in the absolute pressures of the tissue and the blood in the two capillaries. [Note: I originally wrote this as "the difference in the partial pressure of N2 between the tissue and the blood in the two capillaries". See posts below for further discussion.]
Why do pressure differences matter? Pressure is a measure of the average velocity of the gas(es) being measured. How quickly a gas can diffuse into a new volume obviously depends on how quickly it is moving. For diffusion across a permeable membrane, higher pressure means each molecule gets extra shots at finding its way through one of the holes in the membrane over a given period of time.

Molecular diffusion - Wikipedia
Keep in mind that blood flows, constantly exposing fresh blood to my imagined tissue. So I don't have to worry about things like carrying capacity being maxed out in this model. Of course, reality will reduce the effect of everything compared to the simple result in the model, but I don't see how it can overcome the simple physics of diffusion.
I will read and report back@lowwall, I usually hate to appeal to authority, but your contention is contrary to the works of Haldane, Workman, Schreiner, Baker, et. al. This is Baker's introductory comments, highlighting mine to emphasize he says the inert gas pressures, not the total gas pressures:
View attachment 744845
goldfishtornado
Registered
I don't have commentary with respect to the practical implications for diving, but it seems to me the main confusion is between the simple models people use for implementing deco algorithms, heuristics people use thinking about diffusion with respect to diving, and the way real gases behave.
Most of what's been discussed here assumes constant diffusion coefficient, as used in Fick's law. This gives the expression in the "Derivation of Gas Loading Equations" paper. Here, in the simple formulation, each species is treated as independent that's the first expression for Jᵢ in the "alternative formulations." This is the normal partial pressure model people use. In real gasses you need either chemical potential (the second expression for Jᵢ) or the fugacity. If you are very determined you could probably calculate the fugacity from something like the Gibbs energy given in GERG-2004, there's some discussion for other applications of real gas models in this thread.
The real world impact is even worse than that though, because biological membranes do not follow Fick's Law. They are an example of
anomalous diffusion.
There is definitely a difference between the two scenarios, but I'm not sure it matters. The only way to be sure would be an empirical measurement or for someone to at least calculate the order of the effect with a real gas model.
Most of what's been discussed here assumes constant diffusion coefficient, as used in Fick's law. This gives the expression in the "Derivation of Gas Loading Equations" paper. Here, in the simple formulation, each species is treated as independent that's the first expression for Jᵢ in the "alternative formulations." This is the normal partial pressure model people use. In real gasses you need either chemical potential (the second expression for Jᵢ) or the fugacity. If you are very determined you could probably calculate the fugacity from something like the Gibbs energy given in GERG-2004, there's some discussion for other applications of real gas models in this thread.
The real world impact is even worse than that though, because biological membranes do not follow Fick's Law. They are an example of
anomalous diffusion.
There is definitely a difference between the two scenarios, but I'm not sure it matters. The only way to be sure would be an empirical measurement or for someone to at least calculate the order of the effect with a real gas model.
We absolutely agree on the part I bolded. What is the partial pressure of N2 in each of the capillaries?To me the answer is an obvious yes, driven by the difference in the partial pressure of N2 between the tissue and the blood in the two capillaries.
dmaziuk
Contributor
There is definitely a difference between the two scenarios, but I'm not sure it matters.
It doesn't: the point is to get the diver out of the water not bent. The simplified model does it well enough for its definition of "not bent".
You would have to start with a different definition of "not bent" to justify all that extra sophistry.
Similar threads
- Replies
- 135
- Views
- 5,281
- Replies
- 24
- Views
- 6,873
- Replies
- 43
- Views
- 8,441
- Replies
- 5
- Views
- 1,688
- Replies
- 700
- Views
- 66,103



