Curious_George
Green water guy
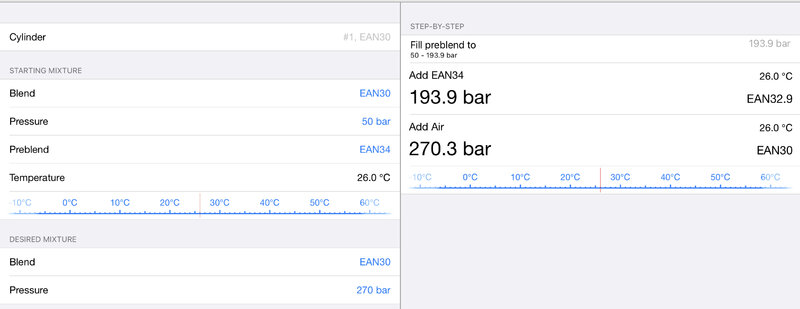
I’m not a technical diver for sure, but do some blending. Appears Baltic Blender will do what you want.
Welcome to ScubaBoard, the world's largest scuba diving community. Registration is not required to read the forums, but we encourage you to join. Joining has its benefits and enables you to participate in the discussions.
Benefits of registering include
Unfortunately it's only available on iphoneI’m not a technical diver for sure, but do some blending. Appears Baltic Blender will do what you want.
View attachment 689730
Sorry to hear that. I use on iPad as well. Anyone who needs an analysis done just send your parameters to me and I’ll give you the recipe.Unfortunately it's only available on iphone
Why? How often are you doing this?I will start a new thread: which software can calculate this.
However, the values of physical quantities as predicted with the Van der Waals equation of state "are in very poor agreement with experiment", so the model's utility is limited to qualitative rather than quantitative purposes.
