Were any OxTox convulsions on old-hands? Just looking for clues why our experiences are so different. Could it be that "most" chamber-experienced commercial/military divers know how to manage CO
2 and perceive OxTox symptoms better? Not sure how it could be tested but wouldn't it be nice if it was that simple? Not talking about eliminating risk, just reducing.
I know there's lots of theories why immersion makes a difference in tolerance, but has anyone actually tested immersed divers in a super-relaxed deep-breathing state? Maybe we need some yoga training... it works wonders for the apniests.

Hello,
This is an important question and I suspect gets to the heart of why Gilliam thinks it is fine to go to 2.8ATA on oxygen underwater - because he has seen it done in a chamber many times without any problems. He is failing to appreciate the difference in risk between a subject at rest in a chamber and a diver underwater that you allude to.
The problem in the water is CO2, and unfortunately, the explanation is so deeply rooted in physiology that while experience may help, no one can avoid the increase in risk no matter how experienced they are.
Perhaps the best place to start is to explain why CO2 levels have a marked tendency to rise when we dive. CO2 is a by-product of oxygen metabolism and we breathe it out. The more gas you move in and out of the lungs the more CO2 you eliminate. Moreover, breathing is largely controlled by CO2 levels, so that if they start to rise, then you breathe more to get rid of it and keep the CO2 levels normal (obviously without thinking about it of course). This control system is extremely well calibrated for a human breathing air at 1 ATA: even if we go running and produce lots of CO2 the breathing will automatically increase enough to keep CO2 levels in the body normal.
This all changes underwater, primarily because an increase in the work of breathing. Work of breathing increases because the gas we are breathing is denser. Just achieving the same flow through the diver's airways takes more work, but then you add underwater breathing apparatus like a rebreather where the diver's effort has to move the gas through hoses, scrubber etc. Being immersed also causes changes in the lungs that increase the work of breathing.
This is where things get really interesting. We have a design flaw (well, at least from a divers perspective) and it affects some more than others, in that when the work of breathing increases many humans will tolerate a rise in CO2 levels rather than perform the extra breathing work required to keep the CO2 normal. This is especially so if you superimpose exercise on top of the increase in work of breathing. The normal CO2 control system becomes insensitive to rising CO2, and there is also evidence that breathing a very high inspired PO2 (as we do during IWR) also worsens this tendency.
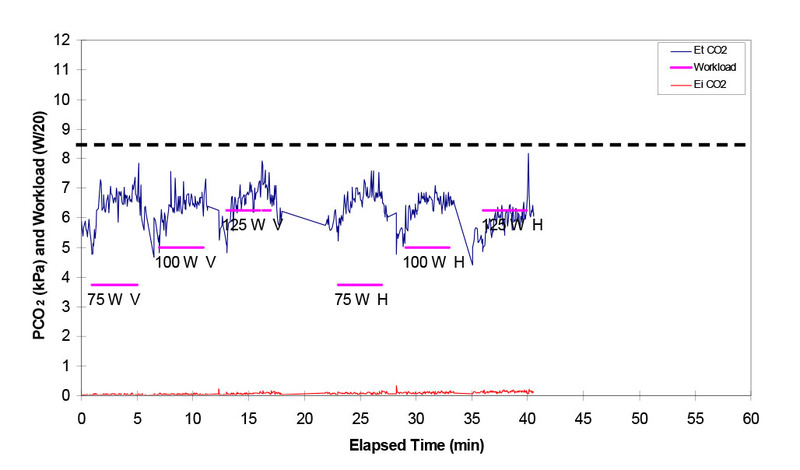
The bottom line is that when underwater with a higher work of breathing and higher inspired PO2, we have a tendency to retain CO2 by not breathing enough, especially if we try to exercise at the same time. This is beautifully illustrated in the figure below from Gavin Anthony's presentation to the DAN Technical Diving Workshop. It shows end tidal CO2 levels (a measure of body CO2 levels) in a diver at 30m on nitrox during performance of intermittent low level exercise (the exercise periods and intensity are shown in the magenta bars). 75W is very low level exercise, probably akin to very gentle finning. The normal level of CO2 is about 5 kPa and the black dashed line at 8.5 kPa indicates a level that is highly dangerous from a CO2 perspective alone. Any rise in CO2 above 5 will increase the oxygen toxicity risk for the reasons explained below. You can see that every time the diver works the CO2 increases and I reiterate that 75 watts is hardly any work. This would not happen in a human exercising (even very hard) at the surface.
OK, so why does increased CO2 increase the risk of cerebral oxygen toxicity?
That was very nicely illustrated by Lambertson and colleagues back in the 1950s. [1] The brain blood flow is very sensitive to CO2 levels. Blood flow normally washes the CO2 produced by the brain away, and if CO2 levels are high, that would normally mean there is not enough blood flow to get rid of the CO2 the brain is producing. Not surprisingly then, the blood vessels of the brain are calibrated to dilate and allow more blood flow if CO2 levels are high. Unfortunately when we are breathing a high PO2 and have high PO2 levels in the blood, this protective mechanism becomes our enemy. The cause of high CO2 in the brain during CO2 retention is not inadequate flow, but rather a failure of the lungs to remove enough CO2 from the blood. Nevertheless, the brain blood vessels don't "know" this and simple do what they are programmed to do when CO2 levels are high - they dilate. The resulting increases in blood flow allows a much bigger delivery of oxygen to the brain. The brain tissue oxygen pressure rises markedly just because the oxygen delivery is much faster, even though the pressure of oxygen inhaled stays the same.
This explains the marked difference in risk of oxygen toxicity between chamber exposure (less work of breathing increase, no immersion, no exercise), and in the water (more work of breathing, immersion and some inevitable degree of low level exercise). Unfortunately, no amount of experience helps with this. Indeed, there is some evidence that experience in diving promotes a tendency to CO2 retention, though the mechanism for this is uncertain.
Bringing this back to the present discussion, this mechanism helps explain why the risk of recompression when immersed breathing denser gas via an underwater breathing apparatus at very high inspired PO2s is so high. All of this is explained in detail in a review article David Doolette and I wrote for the American Physiological Society a few years ago. [2] I would be happy to send it to anyone interested (PM me).
Simon M
1. Lambertsen CJ, Kough RH, Cooper DY, Emmel GL, Loeschcke HH, Schmidt CF. Oxygen toxicity: Effects in man of oxygen inhalation at 1
and 3.5 atmospheres upon blood gas transport, cerebral circulation and cerebral metabolism. J Appl Physiol 1953;5:471-86.
2. Doolette DJ, Mitchell SJ. Hyperbaric conditions. Comprehensive Physiol. 2011;1:163-201.




