Hi all
I have a very basic question about theory.
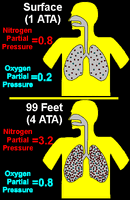
I know the Boyle law, I learned all theory for qualification, but something still bother me: why do we really breathe more at depth?
Of course Boyle law said that air is compressed, but lungs are shrinking too, so you need less air to fill them. Someone told me that lungs are not shrinking the same way because the ribs are protecting them, so the volume is bigger, so more air.
I'm not 100% convinced with that (are the lungs really stuck to the ribs? Couldn't they shrink within the ribs volume?), but this raised another question, what we need is not a "volume" of air, it's a certain number of oxygen molecule, so when air is shrinking we still have the same number of O2 molecules, so we shouldn't need more air...
So, I know the answer is: we breathe more at depth!, but I don't fully understand the theory behind that. Could someone help me?
Thanks a lot
Qasar
I have a very basic question about theory.
I know the Boyle law, I learned all theory for qualification, but something still bother me: why do we really breathe more at depth?
Of course Boyle law said that air is compressed, but lungs are shrinking too, so you need less air to fill them. Someone told me that lungs are not shrinking the same way because the ribs are protecting them, so the volume is bigger, so more air.
I'm not 100% convinced with that (are the lungs really stuck to the ribs? Couldn't they shrink within the ribs volume?), but this raised another question, what we need is not a "volume" of air, it's a certain number of oxygen molecule, so when air is shrinking we still have the same number of O2 molecules, so we shouldn't need more air...
So, I know the answer is: we breathe more at depth!, but I don't fully understand the theory behind that. Could someone help me?
Thanks a lot
Qasar