CuNanoTube
Contributor
Here is a flow diagram I was thinking up.
The Purge system for the CO chamber would probably be the hardest part to plumb, so if the chamber could open the power of one's breath might suffice. I included it because it seems the sensor you found requires an almost stationary air flow which means it might take a long time for all the CO to be pushed out after calibration, especially if the chamber is big. It seems you could greatly reduce the size of the CO analyzer you chose by using a different housing.
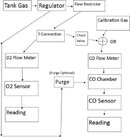
The Purge system for the CO chamber would probably be the hardest part to plumb, so if the chamber could open the power of one's breath might suffice. I included it because it seems the sensor you found requires an almost stationary air flow which means it might take a long time for all the CO to be pushed out after calibration, especially if the chamber is big. It seems you could greatly reduce the size of the CO analyzer you chose by using a different housing.




