Thanks all for the answers. It makes perfect sense as I had not considered that in deco diluent is not used except for flushes.
For those wondering where the hell the question was coming from, it's from this table I built to show what percentage of O2 you need depending on your setpoint as a function of dept. I use it to figure out when the eCCR will struggle to maintain the setpoint (red cells) and hence I would move to the low setpoint (and fly manually). My (wrong) idea was to switch to richer gas when getting shallower to be able to maintain higher pO2 without need for a lot of O2 addition. As many have explained, this doesn't make much sense because you are not adding a lot of diluent in those phases, unless you want to run SCR.
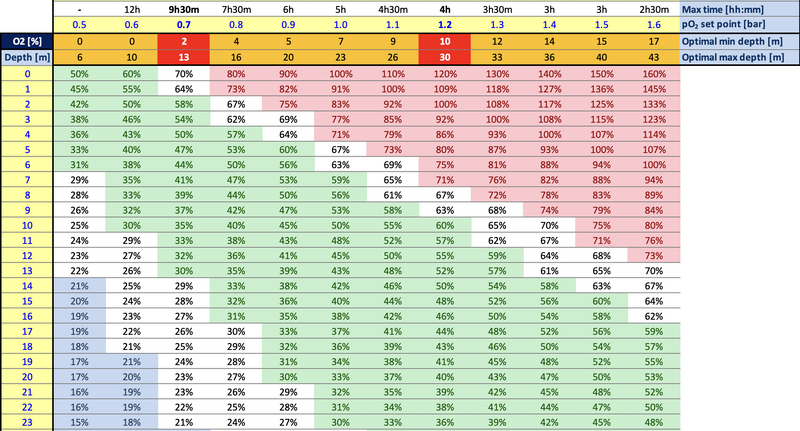
For those wondering where the hell the question was coming from, it's from this table I built to show what percentage of O2 you need depending on your setpoint as a function of dept. I use it to figure out when the eCCR will struggle to maintain the setpoint (red cells) and hence I would move to the low setpoint (and fly manually). My (wrong) idea was to switch to richer gas when getting shallower to be able to maintain higher pO2 without need for a lot of O2 addition. As many have explained, this doesn't make much sense because you are not adding a lot of diluent in those phases, unless you want to run SCR.



